The history of RGTA®
A the late 70s, Denis Barritault was working on aging with Yves Courtois at the INSERM (Institut National de la Santé et la Recherce Médicale). He showed, in collaboration with Michel Pruniéras and Isabelle Saglier-Guedon, that extracts from the retina had proliferative effects on human skin cells in vitro. It was later on proven with Yves Pouliquen that the retina extracts also have re-epithelialization properties in the cornea. These studies led in 1979 to the deposition of the first patents concerning Denis Barritault’s discoveries. As other ocular tissues contained similar activities, the retinal extract was referred to as Eye Derived Growth Factor or EDGF. Denis Barritault made the first assumption of an ubiquitous growth factor in the Eye in 1981 [1]. Other tissues extracted with the same procedure showed similar growth factors properties.and eventually several groups using heparin-affinity chromatography as a purification procedure identified the same molecule. The name FGF was finally adopted for this ubiquitous growth factor at first the meeting gathering all FGF world specialist in 1991 in San Diego.
In 1983, Denis Barritault became full professor at Paris 12 University in Creteil and created with Jean-Pierre Caruelle a new department focused on the understanding of cell growth and tissue repair and regeneration known at the CRRET laboratory (Croissance Cellulaire, Reparation Et Regeneration Tissulaires). This team grew rapidly to develop detection, quantification and purification techniques of FGF and focused on developing FGF as a skin healing agent, in order to improve the healing process and optimize the doses of FGF.
1- Barritault, D., Arruti, C., and Courtois, Y. (1981). Is there a ubiquitous growth factor in the eye? Proliferation induced in different cell types by eye-derived growth factors. Differentiation 18, 29–42. – pubmed
Some candidate polymers were selected as FGF protectors and were tested for wound healing in association with FGF. The unexpected result was to find in the control groups that a heparin-like polymer alone was sufficient to induce quicker and better wound healing than FGF alone or associated with the polymer! No additional FGF was needed. These experiments were repeated several times before Jean-Pierre Caruelle and Denis Barritault were convinced and could make the assumption that the ability of the heparin-like polymer to promote healing by itself may result from the protection of the endogenous FGF. They further assumed that it could also be the result of the protection of other cytokines and growth factors potentially present in the injured tissue and exhibiting high affinity to heparin.
From this initial assumption, a new vision for the organization of the cellular microenvironment and the role of heparan mimetic was suspected. First, the term heparan sulfate mimetic or analogues was preferred to heparin-like as inspired by the natural and ubiquitous heparan sulfate glycosaminoglycan devoid of significant anticoagulant activity. Second, it was postulated that heparan sulfate plays a central role in the regulation of tissue homeostasis, both as a scaffolding element of the extracellular matrix by bridging matrix proteins and as a protector and depot of most communication peptides such as growth factors and cytokines in the extracellular matrix compartment. If today this view is well accepted, it was not in the early 1990s. Third from this lead heparin-like molecule, Denis Barritault and Jean-Pierre Caruelle developed a series of in vitro and in vivo assays and synthesized many polymers with different chemical substitutions to optimize the ability of the heparan sulfate mimetic to perform tissue regeneration. This understanding of the polymer structure needed for regenerating tissue is the heart of the ReGeneraTing Agents technology or RGTA® and the start of a new branch of regenerative medicine known as RGTA®-based Matrix Therapy.
Another demonstration of the heparan mimetic effect was provided with Jean Gautron from the CRRET in a fast and a slow skeletal acute muscle injury adult rat model inducing ischemia, denervation and crush. In this model, fast muscle recovers naturally some function (60%) while slow muscles are transformed only in inert fibrotic tissue with absolute no function (0%) recovery (providing another yes or no model). After administration of heparan mimetic, fast muscle recovered almost 100% while slow muscle recovered 85% of the control lateral healthy muscle function (versus zero in the none treated). Heparan mimetic was able to induce muscle regeneration as shown at histology by muscle fibers organization, revascularisation and neuromuscular junctions with reformation of normal motor end plates and proper molecular weight forms of acetyl choline esterase [4,5]. These results were awarded with the INSERM – French Academy of Sciences prize in 1996 for the best publication of the academy proceedings (Figure 2).
2- Lafont, J., Baroukh, B., Berdal, A., Colombier, M.L., Barritault, D., Caruelle, J.P., and Saffar, J.L. (1998). RGTA11, a new healing agent, triggers developmental events during healing of craniotomy defects in adult rats. Growth Factors 16, 23–38. – pubmed
3- Blanquaert, F., Saffar, J.L., Colombier, M.L., Carpentier, G., Barritault, D., and Caruelle, J.P. (1995). Heparan-like molecules induce the repair of skull defects. Bone 17, 499–506. – pubmed
4- Aamiri, A., Mobarek, A., Carpentier, G., Barritault, D., and Gautron, J. (1995). [Effects of substituted dextran on reinnervation of a skeletal muscle in adult rats during regeneration]. C. R. Acad. Sci. III, Sci. Vie 318, 1037–1043. – pubmed
5- Gautron, J., Kedzia, C., Husmann, I., and Barritault, D. (1995). [Acceleration of the regeneration of skeletal muscles in adult rats by dextran derivatives]. C. R. Acad. Sci. III, Sci. Vie 318, 671–676. – pubmed
Altogether, these works led to the elaboration of the large functionalized polymers, and from that period on, the term RGTA® for ReGeneraTing Agent was coined, protected and preferred to heparan mimetic. A wide network of collaboration aiming to extend these observations from skin to other tissues had started. This led to the deposition of several patents related to the chemical structure, the regeneration process of all tissues that they documented in many models of tissue injuries including skin, mucosa, heart, muscles, vascular and nervous systems, oral and digestive tract, bone, and tendons tissues.
Laureates with top ranking of the first French national competition for the creation of the best innovative company in 1999 (“Concours National de Creation d’Entreprise Innovantes”), Denis Barritault and Jean-Pierre Caruelle created in 2000 the biotechnology company OTR3 (Organ, Tissue, Regeneration, repair, Replacement) that develops medical devices responsible for tissue regeneration.
In 2004 Denis Barritault became the first CEO of OTR3 and left the University as Emeritus professor and honorary director of the CRRET Laboratory.
The life of RGTA® in human health had started.
Chemical structure of RGTA®
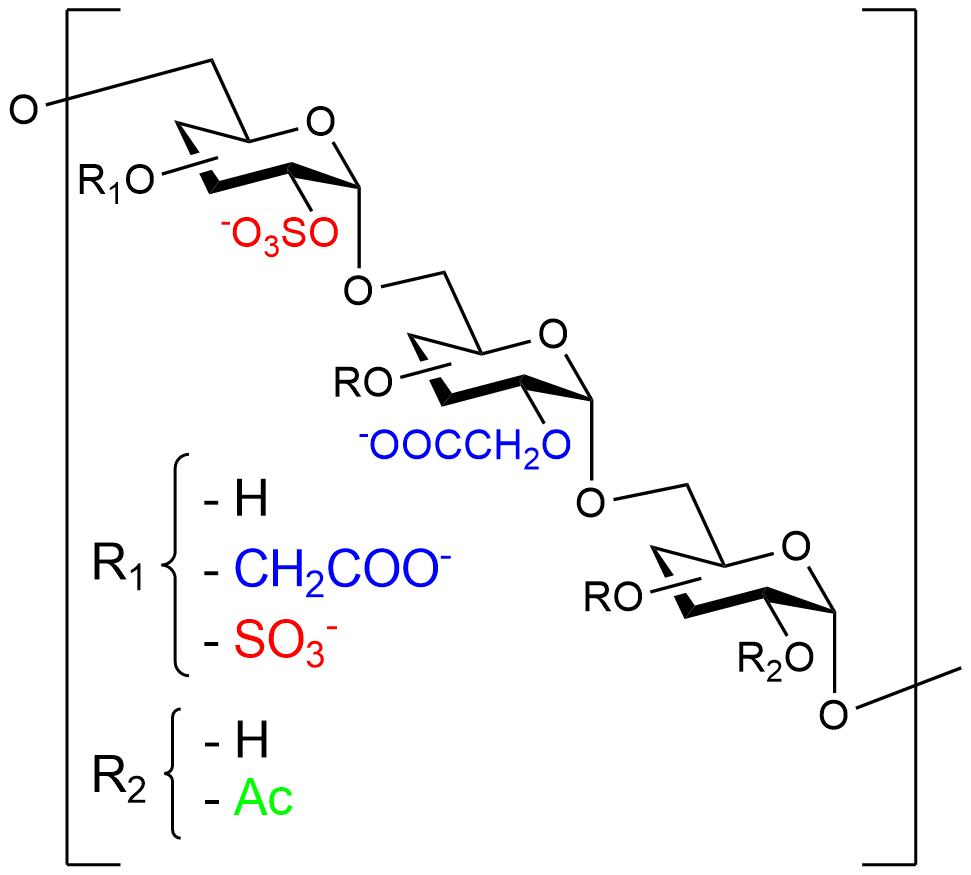
RGTA® are chemically engineered polymers constituted of dextran comprising roughly 250 sugar residues essentially modified with sulfate and carboxyl groups. Due to safety and accessibility, dextran is preferred to other substituted polysaccharides or copolymer backbones such as co-polylactic or malic acid, that could also constitute the RGTA® .
RGTA® mimic natural extracellular matrix heparan sulfate oligosaccharides. Heparan sulfates, that comprise disaccharide subunits linked by β1-4 glycosidic bonds, are prone to degradation by the endoglycosidases. By contrast, RGTA® subunits are linked by α1-6 glycosidic bonds conferring to the compounds resistance to the degradation by endogenous glycosidases.
Mode of action of RGTA®
Illustrated by Elma El Khouri
When introduced in the injured site, the RGTA® technology:
- Replaces degraded Heparan Sulfates
- Binds to proteins of the extracellular matrix such as collagen, elastin, and laminin etc…
- Reconstitutes the matrix architecture and scaffolding
- Becomes an anchorage, storage and protecting site for cellular communication factors or “signals”
- Protects matrix proteins and endogenous factors from degradation by proteases
- Is not degraded by glycanases
- Has no or low anticoagulant activity
RGTA® reconstitutes the extracellular matrix environment allowing damaged tissue to regenerate.
